BICO News Feed
Discover the latest news from our business areas.
- August 6, 2024
- 11:56 am
Hybridoma Technology: Quality Control and Best Practices
Share on facebook
Share on twitter
Share on linkedin
Hybridoma Technology: Quality Control and Best Practices
Hybridoma technology was first described in 1975 by Georges Köhler and Cesar Milstein, who were jointly awarded the Nobel Prize in Physiology or Medicine in 1984 for their discovery (Köhler & Milstein, 1975; The Nobel Prize in Physiology or Medicine 1984, n.d.). It enables the production of monoclonal antibodies by isolating antibody-producing B lymphocytes from animals immunized with the desired antigen. These calls are fused with immortal myeloma cell lines to form hybrid cells, known as hybridomas, which can be cultured in the lab to produce monoclonal antibodies against a specific antigen in high volumes (Mitra & Tomar, 2021; Moraes et al., 2021) (Fig. 1).
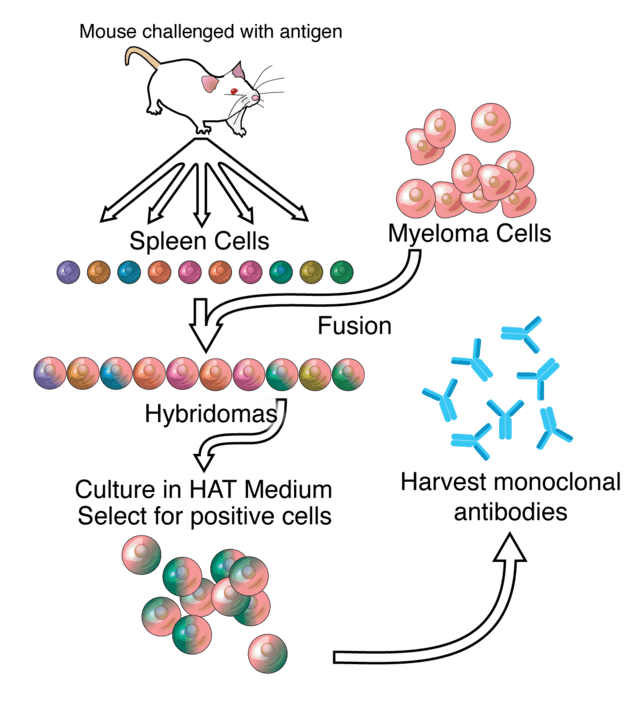
Figure 1. Overview of hybridoma technology and monoclonal antibody creation.
This technology has led to the production of numerous monoclonal antibodies, several of which have been FDA-approved for therapeutic use, such as the mouse monoclonal antibody Muromonab-CD3, approved for kidney transplant rejection (Antibody Therapeutics Approved or in Regulatory Review in the EU or US, n.d.; Todd & Brogden, 1989). More recently, Chinese Hamster Ovary (CHO) cells have become increasingly popular for large-scale antibody production, especially for therapeutic antibodies, due to their ability to perform post-translational modifications resulting in a high yield of stable, properly folded antibodies (Yamaguchi et al., 2023). However, hybridoma technology remains valuable for quickly generating novel antibodies against specific targets, particularly for smaller-scale research applications (Parray et al., 2020).
Due to the importance of hybridoma technology in producing antibodies for research, diagnostic, and therapeutic purposes, ensuring stringent quality control and adhering to best practices is essential (MonoclonalAntibodies, 1999; World Health Organization, 2022). In this article, we will discuss some common challenges, how robust quality control can minimize their impact, and best practices for monoclonal antibody production processes.
Quality Control
Mycoplasma Testing
Mycoplasma contamination is relatively frequent (albeit frustrating) in research and manufacturing laboratories. Mycoplasma are bacteria without cell walls that are small enough to pass through the standard 0.1‐0.22 µm sterilizing‐grade filters used in most labs (Fratz‐Berilla et al., 2020). Mycoplasma generally grow relatively slowly and elicit subtle effects on cell cultures, meaning they often go undetected for extended periods (Nikfarjam & Farzaneh, 2012).
Notwithstanding the costly cleanups required to eliminate mycoplasma contamination, they can also harm the quality of monoclonal antibodies produced in contaminated cells. A recent study demonstrated that contamination of cells with Mycoplasma argini resulted in a significant reduction in antibody product quality (Fratz‐Berilla et al., 2020). This highlights the importance of robust and regular mycoplasma testing in antibody production labs (Mycoplasma Testing, n.d.; World Health Organization, 2022).
The most common mycoplasma testing strategies are:
- Quantitative PCR (qPCR)
- Indirect Hoechst stain
- Culture isolation
Monoclonal Verification
Hybridoma technology faces challenges due to the polyploidy nature of hybridoma cells, which can result in the expression of aberrant immunoglobulin-like transcripts or non-functional antibodies (Babrak et al., 2017). Additionally, hybridomas often express additional functional variable regions, which can complicate the production of truly monoclonal antibodies (Bradbury et al., 2018).
Several verification methods have been developed to address the challenges of hybridoma-derived monoclonal antibody production, including selective PCR followed by tandem mass spectrometry (MS/MS) or next-generation sequencing approaches (Babrak et al., 2017). Moreover, by enabling real-time classification, single-cell dispensing technology (Fig. 2) contributes to more efficient and reliable monoclonal antibody production from hybridoma technology (Riba et al., 2020).

Figure 2. CYTENA’s UP.SIGHT ensures a >99.99% probability of clonal derivation.
Antibody Yield
High antibody yield is essential in monoclonal antibody production and can be maximized with robust quality control. Hybridoma stability issues such as mutations can affect yield and quality. Therefore, identifying and selecting stable clones is essential for maintaining hybridoma stability during production (Castillo et al., 1994). Parameters like pH, temperature, and nutrient supplementation can also significantly influence monoclonal antibody production, and systematic validation of these parameters can support consistent antibody quality and yield. Similarly, optimal cell density and viability are crucial for high monoclonal antibody yield (Wohlenberg et al., 2022).
Ultimately, consistency in laboratory processes is essential for ensuring a high and consistent antibody yield. This can be achieved by integrating automated laboratory tools throughout the process. The UP.SIGHT supports effortless tracking and selection of the highest-producing clones during the cell line development phase, enabling antibody yield to be optimized from the get-go while supporting confluency analysis and cell counting to ensure optimal conditions. It can also be combined with F.QUANT for direct titer quantification from plate supernatant.
Cryopreservation
Maintaining long-term viability and stability of hybridoma cell lines requires proper storage processes; when appropriately stored, hybridoma cell lines can be cryopreserved indefinitely. Hybridoma cells should be suspended in dimethyl sulfoxide (DMSO)/serum, frozen rapidly in a dry ice-ethanol and glycerol bath, and transferred to liquid nitrogen storage. When required, cells can be recovered by thawing rapidly at 37°C, immediately replacing the freezing medium with an appropriate culture medium (Fuller et al., 1988).
Regulatory Compliance
Monoclonal antibodies for diagnostic and therapeutic purposes face strict regulatory hurdles and standards. Meeting these can be made easier by following the quality control and best practices outlined here, as well as by making use of advanced tools that help make proving clonality easier. For example, the UP.SIGHT, an advanced single-cell dispenser and imager with >99.99% probability of clonal derivation, has dual imaging technology that lets scientists easily prove clonality to regulators. Moreover, record-keeping processes must be robust and comprehensive, and built-in imaging technology can support that.
Conclusions and Future Trends
Hybridoma technology is pivotal in monoclonal antibody production for research, diagnostics, and therapeutic applications. Stringent quality control measures, including mycoplasma testing, monoclonal verification, and optimization of antibody yield, are essential for ensuring the reliability and effectiveness of monoclonal antibodies. Best practices, such as proper cryopreservation, meticulous record-keeping, and adherence to regulatory standards, further support high-quality monoclonal antibody production.
Advancements in automation and next-generation sequencing will likely play increasingly important roles in overcoming the challenges associated with hybridoma technology. Automated tools like CYTENA’s UP.SIGHT is already proving invaluable in enhancing process consistency and efficiency. As the demand for monoclonal antibody production continues to grow, ongoing innovation in these areas will be crucial in maintaining the efficiency of hybridoma technology.
CYTENA’s collection of best-in-class instruments allows researchers to streamline their monoclonal antibody production workflows while adhering to best practices and implementing robust quality control.
References
- Antibody therapeutics approved or in regulatory review in the EU or US. (n.d.). The Antibody Society. Retrieved August 22, 2024, from https://www.antibodysociety.org/resources/approved-antibodies/
- Babrak, L., McGarvey, J. A., Stanker, L. H., & Hnasko, R. (2017). Identification and verification of hybridoma-derived monoclonal antibody variable region sequences using recombinant DNA technology and mass spectrometry. Molecular Immunology, 90, 287–294. https://doi.org/10.1016/j.molimm.2017.08.014
- Bradbury, A. R. M., Trinklein, N. D., Thie, H., Wilkinson, I. C., Tandon, A. K., Anderson, S., Bladen, C. L., Jones, B., Aldred, S. F., Bestagno, M., Burrone, O., Maynard, J., Ferrara, F., Trimmer, J. S., Görnemann, J., Glanville, J., Wolf, P., Frenzel, A., Wong, J., … Dübel, S. (2018). When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. mAbs, 10(4), 539–546. https://doi.org/10.1080/19420862.2018.1445456
- Castillo, F. J., Mullen, L. J., Grant, B. C., DeLeon, J., Thrift, J. C., Chang, L. W., Irving, J. M., & Burke, D. J. (1994). Hybridoma stability. Developments in Biological Standardization, 83, 55–64.
- Fratz‐Berilla, E. J., Angart, P., Graham, R. J., Powers, D. N., Mohammad, A., Kohnhorst, C., Faison, T., Velugula‐Yellela, S. R., Trunfio, N., & Agarabi, C. (2020). Impacts on product quality attributes of monoclonal antibodies produced in CHO cell bioreactor cultures during intentional mycoplasma contamination events. Biotechnology and Bioengineering, 117(9), 2802–2815. https://doi.org/10.1002/bit.27436
- Fuller, S. A., Takahashi, M., & Hurrell, J. G. R. (1988). Freezing and Recovery of Hybridoma Cell Lines. Current Protocols in Molecular Biology, 1(1), 11.9.1-11.9.3. https://doi.org/10.1002/0471142727.mb1109s01
- Köhler, G., & Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256(5517), 495–497. https://doi.org/10.1038/256495a0
- Mitra, S., & Tomar, P. C. (2021). Hybridoma technology; advancements, clinical significance, and future aspects. Journal of Genetic Engineering and Biotechnology, 19(1), 159. https://doi.org/10.1186/s43141-021-00264-6
- MonoclonalAntibodies, N. R. C. (US) C. on M. of P. (1999). Large-Scale Production of Monoclonal Antibodies. In Monoclonal Antibody Production. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK100189/
- Moraes, J. Z., Hamaguchi, B., Braggion, C., Speciale, E. R., Cesar, F. B. V., Soares, G. D. F. D. S., Osaki, J. H., Pereira, T. M., & Aguiar, R. B. (2021). Hybridoma technology: Is it still useful? Current Research in Immunology, 2, 32–40. https://doi.org/10.1016/j.crimmu.2021.03.002
- Mycoplasma testing. (n.d.). Culture Collections. Retrieved August 22, 2024, from https://www.culturecollections.org.uk/services/mycoplasma-testing/
- Nikfarjam, L., & Farzaneh, P. (2012). Prevention and detection of Mycoplasma contamination in cell culture. Cell Journal, 13(4), 203–212.
- Parray, H. A., Shukla, S., Samal, S., Shrivastava, T., Ahmed, S., Sharma, C., & Kumar, R. (2020). Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. International Immunopharmacology, 85, 106639. https://doi.org/10.1016/j.intimp.2020.106639
- Riba, J., Schoendube, J., Zimmermann, S., Koltay, P., & Zengerle, R. (2020). Single-cell dispensing and ‘real-time’ cell classification using convolutional neural networks for higher efficiency in single-cell cloning. Scientific Reports, 10(1), 1193. https://doi.org/10.1038/s41598-020-57900-3
- The Nobel Prize in Physiology or Medicine 1984. (n.d.). NobelPrize.Org. Retrieved August 22, 2024, from https://www.nobelprize.org/prizes/medicine/1984/summary/
- Todd, P. A., & Brogden, R. N. (1989). Muromonab CD3. A review of its pharmacology and therapeutic potential. Drugs, 37(6), 871–899. https://doi.org/10.2165/00003495-198937060-00004
- Wohlenberg, O. J., Kortmann, C., Meyer, K. V., Schellenberg, J., Dahlmann, K., Bahnemann, J., Scheper, T., & Solle, D. (2022). Optimization of a mAb production process with regard to robustness and product quality using quality by design principles. Engineering in Life Sciences, 22(7), 484–494. https://doi.org/10.1002/elsc.202100172
- World Health Organization. (2022). Guidelines for the production and quality control of monoclonal antibodies and related products intended for medicinal use. https://cdn.who.int/media/docs/default-source/biologicals/final-who-guidelines-on-mab-production-and-quality-control-annex-4—7-jun-2022.pdf?sfvrsn=8c542f00_1&download=true
- Yamaguchi, K., Ogawa, R., Tsukahara, M., & Kawakami, K. (2023). Efficient production of recombinant proteins in suspension CHO cells culture using the Tol2 transposon system coupled with cycloheximide resistance selection. Scientific Reports, 13(1), 7628. https://doi.org/10.1038/s41598-023-34636-4
Stay updated
Get the latest first.
Subscribe and stay updated with the latest news!


Copyright © 2025 BICO - The Bio Convergence Company - All rights reserved.
Contact us!
This site is protected by reCAPTCHA and the Google
Privacy Policy and
Terms of Service apply.

