BICO News Feed
Discover the latest news from our business areas.
- August 12, 2024
- 12:06 pm
Therapeutic Monoclonal Antibody Applications
Share on facebook
Share on twitter
Share on linkedin
Therapeutic Monoclonal Antibody Applications
Monoclonal antibodies are highly specific antibodies designed to bind to a particular epitope on an antigen with high affinity (Castelli et al., 2019). Monoclonal antibodies are produced using hybridoma technology or Chinese Hamster Ovary (CHO) cells. Hybridoma technology was introduced by Köhler and Milstein in 1975 and involves the fusion of antibody-producing B cells with immortal myeloma cells to create hybridomas that can be cultured to produce large quantities of monoclonal antibodies (Köhler & Milstein, 1975; Mitra & Tomar, 2021). The first FDA-approved monoclonal antibody therapy, Muromonab-CD3, was produced by hybridoma technology and approved in 1986 (Todd & Brogden, 1989). Later, researchers began exploring CHO cells for recombinant protein production, and the first FDA-approved therapeutic monoclonal antibody produced in CHO cells was Rituximab in 1997 (Zhang et al., 2022).
Nowadays, CHO cells represent the industry standard for therapeutic antibody production due to their ability to perform post-translational modifications, resulting in a high yield of stable, properly folded antibodies (Yamaguchi et al., 2023). However, hybridoma technology remains a valuable tool and is often preferred for producing antibodies for research applications due to its faster production timelines and ability to preserve native antibody structures (Parray et al., 2020).
Since their discovery, monoclonal antibodies have proven invaluable for various therapeutic applications (Mekala et al., 2024). In this article, we will delve into these applications, explore the challenges faced in producing monoclonal antibodies for therapeutic uses, and highlight strategies to overcome these hurdles.
Therapeutic Uses of Monoclonal Antibodies
Cancer
Targeted Therapy
In cancer therapy, monoclonal antibodies target specific antigens on tumor cells to inhibit growth and induce cell death. Trastuzumab (Herceptin) was the first monoclonal antibody therapy approved for HER2-positive breast cancer (Trastuzumab, n.d.). It works by stimulating antibody-dependent cell-mediated cytotoxicity of HER2-expressing cells (Namboodiri & Pandey, 2011). Trastuzumab has now been approved for several other indications, including HER-positive gastric cancer, non-small cell lung cancer, and colorectal cancer (Greenblatt & Khaddour, 2024).
Other notable monoclonal antibody therapies for cancer include cetuximab (Erbitux) and bevacizumab (Avastin), which target EGFR and VEGF, respectively, and have both demonstrated clinical value in metastatic colorectal cancer, among other cancers (Chidharla et al., 2024; Gerriets & Kasi, 2024).
Immune Checkpoint Inhibitors
Recently, immune checkpoint inhibitors (ICIs) have emerged as a powerful class of monoclonal antibody therapeutics that block immune checkpoint molecules, enhancing the immune response against tumors. The FDA has approved three categories of ICIs: PD-1, PDL-1, and CTLA-4 inhibitors (Shiravand et al., 2022).
Antibody-Drug Conjugates
Monoclonal antibodies’ applications also include antibody-drug conjugates (ADCs; Fig. 1), where they are chemically linked to a cytotoxic drug to deliver chemotherapeutics directly to cancer cells (Fu et al., 2022). The first ADC, gemtuzumab ozogamicin (Mylotarg), was approved in 2000 for treating CD33-positive acute myeloid leukemia, with many others approved or investigated in clinical trials since (Fu et al., 2022; Swaminathan & Cortes, 2023).
Figure 1. Antibody-drug conjugates are targeted medicines that deliver chemotherapy agents to cancer cells.
Immune System Disorders
Autoimmune and Inflammatory Diseases
The ability of monoclonal antibodies to target immune pathways makes them a promising therapeutic strategy for autoimmune and inflammatory conditions. For example, anti-TNF-α antibodies such as infliximab and adalimumab effectively manage rheumatoid arthritis and inflammatory bowel disease (Felten et al., 2023; Slevin & Egan, 2015). These antibodies bind to TNF-α, preventing it from triggering inflammatory cascades and alleviating symptoms caused by excessive inflammation (Berger et al., 2002). Additionally, monoclonal antibodies targeting interleukins have been developed to treat psoriasis (Jeon et al., 2017).
Allergies
Monoclonal antibodies can modulate the immune response to allergens, increasing the threshold of tolerated doses and reducing the risk of serious reactions such as anaphylaxis, improving outcomes and potentially saving lives in patients with severe allergies (Manti et al., 2021).
Transplant Medicine
Monoclonal antibodies used to manage transplant rejection typically target specific CD proteins on T or B cells, such as CD3, CD25, and CD52, improving tolerability and reversing rejection in transplant recipients (Costanzo, 1996; Zaza et al., 2014). Other monoclonal antibody therapies have been explored to manage post-transplant complications such as cytomegalovirus infection and lymphoproliferative disease (Ishida et al., 2017; Pescovitz, 2004).
Infectious Diseases
Neutralizing antibodies can target specific viral epitopes, blocking viral entry and replication. Monoclonal antibodies can be used pre-exposure to prevent infection, post-exposure to reduce infection severity, or as a treatment. They have been investigated for the prevention or treatment of respiratory syncytial virus (RSV), COVID-19, influenza, malaria, and Ebola (Otsubo & Yasui, 2022).
Challenges in Therapeutic Uses of Monoclonal Antibodies
While therapeutic monoclonal antibody applications have proven revolutionary, monoclonal antibody production still faces significant challenges that complicate the manufacturing process and limit accessibility (Sifniotis et al., 2019). The complexity of scaling up production while maintaining quality, consistency, and yield is a significant hurdle; ensuring product quality and consistency across batches requires robust testing, while maintaining genetic stability in production cell lines poses a further challenge. Moreover, monoclonal antibodies still carry the risk of eliciting immune responses in patients and navigating the regulatory approval process can be complex.
Overcoming these obstacles necessitates continuous research and technological advancements (Sifniotis et al., 2019). Tools like single-cell dispensers can significantly enhance monoclonal antibody production by improving cell line development, ensuring consistency, and reducing production time and costs (Fig. 2). They enable precise isolation of high-yield, genetically stable clones, which streamlines the development process and enhances product quality.
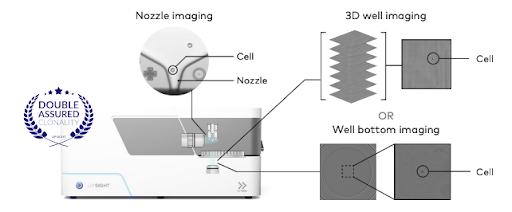
Figure 2. The UP.SIGHT’s dual-imaging technology results in >99.99% probability of clonality, ensuring that your monoclonal antibody product meets the strict regulatory standards for product consistency and reproducibility.
Conclusions and Future Trends
While monoclonal antibody applications continue to revolutionize the treatment of various diseases, their production still faces significant challenges, particularly in scaling up manufacturing while maintaining quality and consistency. Future advancements will likely focus on optimizing production processes, improving the stability and efficacy of monoclonal antibodies, and reducing costs to enhance accessibility. Tools like single-cell dispensers offer promising solutions by streamlining cell line development and ensuring product consistency, paving the way for more efficient and scalable production of monoclonal antibodies for therapeutic use.
CYTENA’s collection of best-in-class instruments allows researchers to streamline their monoclonal antibody production workflows for therapeutic monoclonal antibody applications.
References
-
- Berger, M., Shankar, V., & Vafai, A. (2002). Therapeutic Applications of Monoclonal Antibodies. The American Journal of the Medical Sciences, 324(1), 14–30. https://doi.org/10.1097/00000441-200207000-00004
- Castelli, M. S., McGonigle, P., & Hornby, P. J. (2019). The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacology Research & Perspectives, 7(6), e00535. https://doi.org/10.1002/prp2.535
- Chidharla, A., Parsi, M., & Kasi, A. (2024). Cetuximab. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK459293/
- Costanzo, M. R. (1996). New monoclonal antibodies. Current Opinion in Cardiology, 11(2). https://doi.org/10.1097/00001573-199603000-00014
- Felten, R., Mertz, P., Sebbag, E., Scherlinger, M., & Arnaud, L. (2023). Novel therapeutic strategies for autoimmune and inflammatory rheumatic diseases. Drug Discovery Today, 28(7), 103612. https://doi.org/10.1016/j.drudis.2023.103612
- Fu, Z., Li, S., Han, S., Shi, C., & Zhang, Y. (2022). Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduction and Targeted Therapy, 7(1), 1–25. https://doi.org/10.1038/s41392-022-00947-7
- Gerriets, V., & Kasi, A. (2024). Bevacizumab. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK482126/
- Greenblatt, K., & Khaddour, K. (2024). Trastuzumab. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK532246/
- Ishida, J. H., Patel, A., Mehta, A. K., Gatault, P., McBride, J. M., Burgess, T., Derby, M. A., Snydman, D. R., Emu, B., Feierbach, B., Fouts, A. E., Maia, M., Deng, R., Rosenberger, C. M., Gennaro, L. A., Striano, N. S., Liao, X. C., & Tavel, J. A. (2017). Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of RG7667, a Combination Monoclonal Antibody, for Prevention of Cytomegalovirus Infection in High-Risk Kidney Transplant Recipients. Antimicrobial Agents and Chemotherapy, 61(2), e01794-16. https://doi.org/10.1128/AAC.01794-16
- Jeon, C., Sekhon, S., Yan, D., Afifi, L., Nakamura, M., & Bhutani, T. (2017). Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Human Vaccines & Immunotherapeutics, 13(10), 2247–2259. https://doi.org/10.1080/21645515.2017.1356498
- Köhler, G., & Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256(5517), 495–497. https://doi.org/10.1038/256495a0
- Manti, S., Pecora, G., Patanè, F., Giallongo, A., Parisi, G. F., Papale, M., Licari, A., Marseglia, G. L., & Leonardi, S. (2021). Monoclonal Antibodies in Treating Food Allergy: A New Therapeutic Horizon. Nutrients, 13(7), 2314. https://doi.org/10.3390/nu13072314
- Mekala, J. R., Nalluri, H. P., Reddy, P. N., S.b., S., N.s., S. K., G.v.s.d., S. K., Dhiman, R., Chamarthy, S., Komaragiri, R. R., Manyam, R. R., & Dirisala, V. R. (2024). Emerging trends and therapeutic applications of monoclonal antibodies. Gene, 925, 148607. https://doi.org/10.1016/j.gene.2024.148607
- Mitra, S., & Tomar, P. C. (2021). Hybridoma technology; advancements, clinical significance, and future aspects. Journal of Genetic Engineering and Biotechnology, 19(1), 159. https://doi.org/10.1186/s43141-021-00264-6
- Namboodiri, A. M., & Pandey, J. P. (2011). Differential inhibition of trastuzumab- and cetuximab-induced cytotoxicity of cancer cells by immunoglobulin G1 expressing different GM allotypes. Clinical and Experimental Immunology, 166(3), 361–365. https://doi.org/10.1111/j.1365-2249.2011.04477.x
- Otsubo, R., & Yasui, T. (2022). Monoclonal antibody therapeutics for infectious diseases: Beyond normal human immunoglobulin. Pharmacology & Therapeutics, 240, 108233. https://doi.org/10.1016/j.pharmthera.2022.108233
- Parray, H. A., Shukla, S., Samal, S., Shrivastava, T., Ahmed, S., Sharma, C., & Kumar, R. (2020). Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. International Immunopharmacology, 85, 106639. https://doi.org/10.1016/j.intimp.2020.106639
- Pescovitz, M. D. (2004). The use of rituximab, anti-CD20 monoclonal antibody, in pediatric transplantation. Pediatric Transplantation, 8(1), 9–21. https://doi.org/10.1046/j.1397-3142.2003.00135.x
- Shiravand, Y., Khodadadi, F., Kashani, S. M. A., Hosseini-Fard, S. R., Hosseini, S., Sadeghirad, H., Ladwa, R., O’Byrne, K., & Kulasinghe, A. (2022). Immune Checkpoint Inhibitors in Cancer Therapy. Current Oncology, 29(5), 3044–3060. https://doi.org/10.3390/curroncol29050247
- Sifniotis, V., Cruz, E., Eroglu, B., & Kayser, V. (2019). Current Advancements in Addressing Key Challenges of Therapeutic Antibody Design, Manufacture, and Formulation. Antibodies, 8(2), 36. https://doi.org/10.3390/antib8020036
- Slevin, S. M., & Egan, L. J. (2015). New Insights into the Mechanisms of Action of Anti-Tumor Necrosis Factor-α Monoclonal Antibodies in Inflammatory Bowel Disease. Inflammatory Bowel Diseases, 21(12), 2909–2920. https://doi.org/10.1097/MIB.0000000000000533
- Swaminathan, M., & Cortes, J. E. (2023). Update on the role of gemtuzumab-ozogamicin in the treatment of acute myeloid leukemia. Therapeutic Advances in Hematology, 14, 20406207231154708. https://doi.org/10.1177/20406207231154708
- Todd, P. A., & Brogden, R. N. (1989). Muromonab CD3: A Review of its Pharmacology and Therapeutic Potential. Drugs, 37(6), 871–899. https://doi.org/10.2165/00003495-198937060-00004
- Trastuzumab. (n.d.). Retrieved August 22, 2024, from https://www.cancerresearchuk.org/about-cancer/treatment/drugs/trastuzumab
- Yamaguchi, K., Ogawa, R., Tsukahara, M., & Kawakami, K. (2023). Efficient production of recombinant proteins in suspension CHO cells culture using the Tol2 transposon system coupled with cycloheximide resistance selection. Scientific Reports, 13(1), 7628. https://doi.org/10.1038/s41598-023-34636-4
- Zaza, G., Tomei, P., Granata, S., Boschiero, L., & Lupo, A. (2014). Monoclonal Antibody Therapy and Renal Transplantation: Focus on Adverse Effects. Toxins, 6(3), 869. https://doi.org/10.3390/toxins6030869
- Zhang, J.-H., Shan, L.-L., Liang, F., Du, C.-Y., & Li, J.-J. (2022). Strategies and Considerations for Improving Recombinant Antibody Production and Quality in Chinese Hamster Ovary Cells. Frontiers in Bioengineering and Biotechnology, 10, 856049. https://doi.org/10.3389/fbioe.2022.856049
More news
Stay updated
Get the latest first.
Subscribe and stay updated with the latest news!

Copyright © 2026 BICO - All rights reserved.
Contact us!
This site is protected by reCAPTCHA and the Google
Privacy Policy and
Terms of Service apply.

