BICO News Feed
- September 23, 2024
- 10:10 am
Unlocking the Future of Medicine: The Promise and Techniques of Allogeneic Stem Cell Therapies
Figure 1. Stem cell therapy is used to treat conditions like neurodegenerative and cardiovascular disease.
Therapeutic Potential of Allogeneic Stem Cell Therapy
Stem cell therapies have the potential to treat a wide range of diseases, especially in the field of regenerative medicine1. They also have critical applications in research and development.
- Disease Modeling – The versatility of iPSC techniques makes them fantastic for generating models of a massive range of diseases3. Furthermore, they can be genetically modified to express disease-associated genetic variants4.
- Drug Discovery – iPSCs are used to generate cells that more closely resemble disease conditions, enabling more accurate testing of potential drugs5. For instance, iPSCs can be differentiated into neurons and modified to express genetic variants associated with Alzheimer’s disease6. Researchers can use these cells in high-throughput assays to test how potential drugs affect the disease mechanisms and neuronal function.
Medicine
Advantages
- Universality – Allogeneic stem cell therapy can theoretically be given to any individual, making them an “off-the-shelf” solution for several diseases for which there is an urgent medical need7.
- Scalability – Their universality makes these cells suitable for large-scale production, leading to cost savings and improved standardization8.
Challenges
- Immune Rejection – While these cells are often modified to overcome immune rejection, it remains challenging to apply them as therapies in some instances9.
- Tumorigenicity – iPSC-based therapies can carry a risk of tumor development in patients, though this can also be reduced via genetic modifications10.
- Cost – These therapies are relatively expensive to produce, though allogeneic therapies benefit from economies of scale that autologous therapies do not11.
We have only scratched the surface of how allogeneic stem cells can transform regenerative medicine. For a more detailed discussion read our full article on this topic.
Core Methods
Generating iPSC-based allogeneic cell therapies is a long and complicated process, and researchers must overcome many challenges and potential pitfalls to produce a high quality stem cell therapy.
Process
- Reprogramming and Modification – Somatic cells are dedifferentiated to a pluripotent state by the addition of transcription factors known collectively as Yamanaka factors12.
- Selection – Single iPSCs are seeded into individual wells to facilitate the growth of monoclonal populations. The UP.SIGHT from CYTENA provides gentle dispensing and >97% single-cell dispensing efficiency. This application note demonstrates how the UP.SIGHT addresses common challenges in iPSC dispensing and cultivation.
- Quality Control (QC) – Robust QC processes control for potential issues like loss of pluripotency, contamination, and genomic instability. QC is performed throughout the development process13.
- Scaling – Scaling up occurs both at the iPSC stage and after iPSCs have been differentiated so there is a stock of differentiated cells ready for clinical use.
- Storing – iPSCs are typically cryopreserved at 196°C with intermediate cooling steps14. Improper storage can affect downstream function.
Therapy Success Story - Aloficel
Future Technologies
3D printing technologies allow iPSCs to be integrated into biomaterials and organoids, providing better disease models and functional cell-based therapeutics for tissue regeneration. Organoids are particularly promising for use in the early stages of drug development to better recapitulate complex diseases (Fig. 2)18.

Figure 2. Organoids offer a more realistic model for disease than monoclonal cell lines.
For more information on how iPSC-based therapies are produced and the associated challenges, read our full article, which covers these topics in more detail.
Benefits of Automation in iPSC Therapy Production
Automation confers numerous benefits that streamline production and enhance the quality of the final product.
Efficiency and Scale
Higher Throughput
Liquid handlers that use automation can dispense cells and media more accurately and quickly than manual methods. This means more cells can be seeded in the same workflow. When coupled with dedicated software like CYTENA’s C.STUDIO, automation makes it simple to screen thousands of clones and identify the best ones to proceed with.
Regulatory Compliance
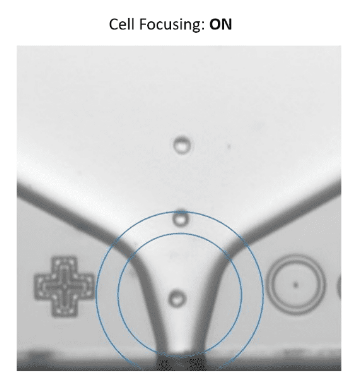
Figure 3. The UP.SIGHT makes it easy to prove monoclonality to regulators and gives researchers confidence in their cell line development workflows.
For more information on this topic, including how automation reduces the risk of contamination and improves workflow reliability, read our full-length article.
Conclusion
Allogeneic stem cell therapy holds immense promise in transforming regenerative medicine and drug discovery. By leveraging iPSCs, these therapies offer versatile solutions for treating various diseases. We created a tracker to monitor the cell and gene therapy pipeline, highlighting clinical trial progress and regulatory approvals. Automation helps researchers overcome common hurdles in therapy development by streamlining production processes, improving efficiency, and facilitating regulatory compliance. As technology advances, the future of allogeneic stem cell therapies is becoming even brighter, opening up new opportunities for personalized medicine and large-scale treatments.
CYTENA’s suite of high-end instrumentation supports ambitious researchers striving to produce the next generation of regenerative medicines. Contact our team to learn more about the UP.SIGHT single cell dispenser. Book a demo to explore its capabilities first hand.
References
- Aboul-Soud MAM, Alzahrani AJ, Mahmoud A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells. 2021;10(9):2319. doi:10.3390/cells10092319
- Doulgkeroglou MN, Di Nubila A, Niessing B, et al. Automation, Monitoring, and Standardization of Cell Product Manufacturing. Front Bioeng Biotechnol. 2020;8:811. doi:10.3389/fbioe.2020.00811
- Liu C, Oikonomopoulos A, Sayed N, Wu JC. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development. 2018;145(5):dev156166. doi:10.1242/dev.156166
- Nicholson MW, Ting CY, Chan DZH, et al. Utility of iPSC-Derived Cells for Disease Modeling, Drug Development, and Cell Therapy. Cells. 2022;11(11):1853. doi:10.3390/cells11111853
- Elitt MS, Barbar L, Tesar PJ. Drug screening for human genetic diseases using iPSC models. Human Molecular Genetics. 2018;27(R2):R89-R98. doi:10.1093/hmg/ddy186
- Penney J, Ralvenius WT, Tsai LH. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol Psychiatry. 2020;25(1):148-167. doi:10.1038/s41380-019-0468-3
- González BJ, Creusot RJ, Sykes M, Egli D. How Safe Are Universal Pluripotent Stem Cells? Cell Stem Cell. 2020;26(3):307-308. doi:10.1016/j.stem.2020.02.006
- Jha BS, Farnoodian M, Bharti K. Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells-based therapy product. Stem Cells Transl Med. 2021;10(2):198-208. doi:10.1002/sctm.20-0242
- Petrus-Reurer S, Romano M, Howlett S, Jones JL, Lombardi G, Saeb-Parsy K. Immunological considerations and challenges for regenerative cellular therapies. Commun Biol. 2021;4(1):798. doi:10.1038/s42003-021-02237-4
- Sato Y, Bando H, Di Piazza M, et al. Tumorigenicity assessment of cell therapy products: The need for global consensus and points to consider. Cytotherapy. 2019;21(11):1095-1111. doi:10.1016/j.jcyt.2019.10.001
- McKenna DH, Perlingeiro RCR. Development of allogeneic iPS cell-based therapy: from bench to bedside. EMBO Mol Med. 2023;15(2):e15315. doi:10.15252/emmm.202115315
- Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Sig Transduct Target Ther. 2024;9(1):112. doi:10.1038/s41392-024-01809-0
- Madrid M, Lakshmipathy U, Zhang X, et al. Considerations for the development of iPSC-derived cell therapies: a review of key challenges by the JSRM-ISCT iPSC Committee. Cytotherapy. Published online June 2024:S1465324924007308. doi:10.1016/j.jcyt.2024.05.022
- Yuan Y, Yang Y, Tian Y, et al. Efficient long-term cryopreservation of pluripotent stem cells at −80 °C. Sci Rep. 2016;6(1):34476. doi:10.1038/srep34476
- Lee JL, Yoon YS, Yu CS. Treatment Strategy for Perianal Fistulas in Crohn Disease Patients: The Surgeon’s Point of View. Ann Coloproctol. 2021;37(1):5-15. doi:10.3393/ac.2021.02.08
- Mezey É. Human Mesenchymal Stem/Stromal Cells in Immune Regulation and Therapy. Stem Cells Translational Medicine. 2022;11(2):114-134. doi:10.1093/stcltm/szab020
- Takeda Receives Approval to Manufacture and Market Alofisel®▼ (darvadstrocel) in Japan for Treatment of Complex Perianal Fistulas in Patients with Non-active or Mildly Active Luminal Crohn’s Disease. Accessed September 30, 2024. https://www.takeda.com/newsroom/newsreleases/2021/takeda-receives-approval-to-manufacture-and-market-alofisel-darvadstrocel-in-japan-for-treatment-of-complex-perianal-fistulas-in-patients-with-non-active-or-mildly-active-luminal-crohns-disease/
- Soman SS, Vijayavenkataraman S. Applications of 3D Bioprinted-Induced Pluripotent Stem Cells in Healthcare. Int J Bioprint. 2020;6(4):280. doi:10.18063/ijb.v6i4.280
- Holland I, Davies JA. Automation in the Life Science Research Laboratory. Front Bioeng Biotechnol. 2020;8(571777). doi:10.3389/fbioe.2020.571777
Stay updated
Get the latest first.