The National Institutes of Health (NIH) in the United States has recently followed the FDA’s lead in issuing guidelines to reduce animal testing in laboratory research. This significant shift in the regulatory landscape strongly aligns with BICO’s strategic position in the advanced 3D cell model market and our ambition to end animal testing.
“We are confident that the regulatory developments seen in the US validate our continued development of advanced 3D cell model technologies and support our commitment to ending animal testing. We look forward to providing updates on our progress, workflow examples, and real-life case studies soon.” – Anders Fogelberg, CCO, BICO
Capabilities in the BICO Portfolio
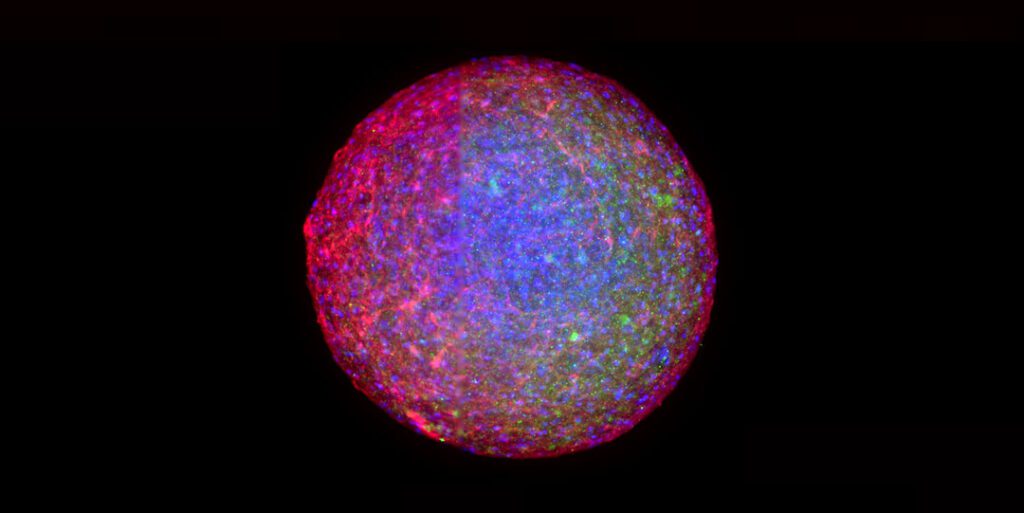
New Approach Methodologies (NAMs) represent potential alternatives to animal testing in the non-clinical development phase of new medicines. BICO offers solutions for NAM models tailored for nearly all aspects of the workflow from preparation to analysis.
Advanced BioMatrix: Large portfolio of high-quality, defined ECMs and tunable hydrogels
Biosero: Use Green Button Go to automate steps in the 3D cell model workflow
Cellenion: Precise organoid and spheroid isolation and sorting
CELLINK: 3D bioprinting for the fabrication of biomimetic tissue models
DISPENDIX: Liquid handling in miniaturized drug assay formats
ECHO: Microscopes and live-cell imaging for real-time monitoring and analysis
An exciting trend is that more and more researchers utilize the synergies within the BICO product portfolio, enabling both better 3D cell models and a reduction in the use of animal models. This trend aligns with the increasing adoption of 3D cell culture methods. Since 2021, the number of publications focused on 3D cell culture has by far outpaced 2D cell culture.
Learn More
Reach out if you are interested in learning more about 3D cell models. You can find links to a selection of our solutions below: