Kundcase

Ny teknik för att snabbare upptäcka urinblåsecancer
SCIENION utvecklar tillsammans med Institutet för förebyggande och arbetsmedicin (IPA) en icke-invasiv detektion av cancer i urinblåsan för att förbättra patienters överlevnadsgrad och livskvalitet.
Automatiserad proteinrening som påskyndar upptäckten av antikroppar
Biosero hjälpte nyligen ett immunonkologiföretag att helt automatisera proteinrening. Detta frigjorde mycket tid för labbpersonalen, minskade antalet mänskliga fel och kostnader samt påskyndade antikroppsupptäcktens arbetsflöde.
Identifiering av bakterier för att motverka antibiotikaresistens
Antibiotikaresistens är ett av de största hoten mot global hälsa, livsmedelssäkerhet och utveckling. Med detta i åtanke utvecklade forskare vid Imperial College London en ny fiberoptisk Raman-spektroskopisensor för etikettfri identifiering av bakterier.
Potentiell behandling för degenerativa muskelsjukdomar
Forskare vid Johns Hopkins University genomförde en studie på mänskliga stamceller och dess potentiella användning som behandling för degenerativa muskelsjukdomar.

3D bioprintade plåster som behandling för diabetespatienter med fotsår
CELLINKs bioprinter BIO X hjälper till att påskynda läkningen av diabetiska fotsår (DFU) med 3D bioprintade plåster som möjliggör fördröjd frisättning av antibiotika direkt till sårområdet.

Personaliserad vård med bioprintat brosktransplantat
CELLINKs INKREDIBLE+™-bioprinter och Advanced BioMatrixs LifeInk® 200 typ I-kollagen användes i en banbrytande bioprintningsstudie för näsbrosk som nyligen publicerades i Journal of Tissue Engineering.

3D bioprinting av brosk med iPS-celler för att utveckla behandlingar för knäskador
En forskargrupp vid Göteborgs universitet har utvecklat en fördjupad förståelse för stamceller och hur de kan omprogrammeras för att skapa livsförändrande terapier.
Bioprintade tumörmodeller för individualiserad läkemedelstestning
Carcinotech bioprintar patientspecifika testmodeller med celler från biopsiprover och har dragit stor nytta av CELLINKs expertis inom området.

Tester som upptäcker gifter i dricksvattnet
SCIENION var en av 12 partners i det EU-finansierade projektet microAQUA där den allmänna vattenförsörjningen testades och analyserades för att upptäcka smittoämnen och gifter.

Exakta och känsliga verktyg för för övervakning av COVID-19
Karolinska Institutet utförde en studie där DISPENDIX vätskehanterare I.DOT användes för att automatisera och effektivisera COVseq- tekniken för att framställa multiplexa bibliotek för DNA sekvensering.

Bioprintad hjärtvävnad som minimerar risken för transplantationsavstötning
University of Technology i Sydney utvecklar bioprintad hjärtvävnad med patientens egna stamceller på begäran för att minimera risken för transplantationsavstötning.

Massproducerade stamceller som inkörsport till personaliserad medicin
Ronawk massproducerar adulta stamceller med hjälp av bioprintrar från CELLINK vilket banar väg för att i framtiden kunna odla och transplantera patienters egna stamceller utan att behöva de immunsuppressiva läkemedel som vanligtvis krävs efter en organdonation.

Relevanta alternativ till djurförsök vid test av kvinnlig intimvård
Företaget Good Clean Love använder MatTeks 3D-modell av mänsklig vävnad istället för djurförsök vid testning av kvinnliga intimvårdsprodukter.
Ett steg närmare implanterbara hjärtplåster
Forskare från Boston University har tillverkat ett mikrofluidisk hjärta-på-ett chip, en modell av ett mänskligt hjärta vilket banar väg för att så småningom möjliggöra tillverkning av vävnad som kan implanteras i hjärtat.

Mer kostnadseffektiv och storskalig tillverkning av snabbtester
Ginolis hjälper Abingdon Health med flexibla lösningar för att producera snabbtester i kraftigt ökade volymer till lägre priser.

Mer effektiv kemoterapi för hjärntumörer
Cornell Medical College utvecklar nanomaterial för mer effektiv levererans av onkologiska läkemedel till hjärnan, eliminera cancertumörer och i slutändan förbättra överlevnaden för patienter med DIPG- och andra hjärntumörer.

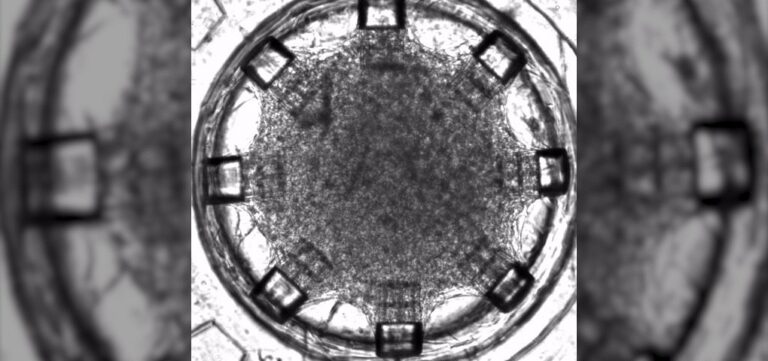
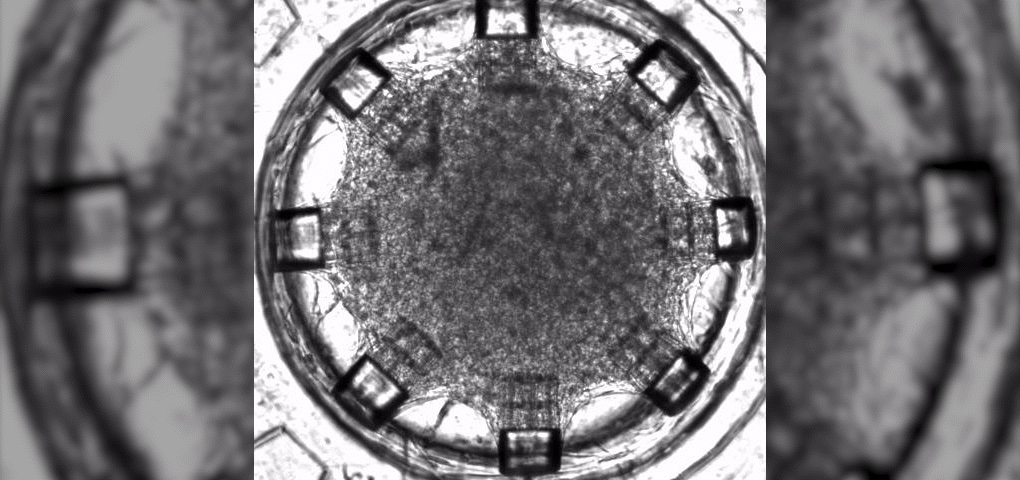
Using the CYTENA UP.SIGHT, Dr. Rafal Krol optimized and automated processes in single-cell cloning and cell line development stability. The purpose was to produce monoclonal antibodies, viral vectors and iPSC cloning for cell therapy.
”With the nozzle images, we can reliably see for each cell being dispensed, the size and roundness and we have this [information] before we start dispensing… That’s really very beneficial for the UP.SIGHT and I can say that this technological advancement is really huge.”
Dr. Rafal Krol
Chief Research Scientist CiRA Foundation

US based InBios, a leading biotechnology company, utilized eight Ginolis Lateral Flow Device Assembly (LFDA) semi-automated
and automated systems in a series. By doing this InBios upscaled production of rapid antigen test kits 20-fold, which enabled them to produce kits at a scale needed to contribute significantly to emergency demands for testing.


Diffuse intrinsic pontine gliomas (DIPG) are inoperable brain tumors located in the pons, a region of the brain stem that regulates critical functions like respiration, chewing and swallowing. And every year, approximately 150 to 300 children, largely under the age of 10, are diagnosed with DIPG tumors in the United States. Unfortunately, DIPG’s aggressiveness means that the median survival is less than a year after diagnosis, and what limited treatment options are available extend survival for less than 10% of patients and by just a few years.
Dr. Benedict Law, an associate professor of pharmacology in the Radiology Department at the Weill Cornell Medical College, is developing nanomaterials to more efficiently deliver oncology drugs to the brain, eliminate cancerous tumors and, ultimately, improve the survival rate of patients with DIPG tumors among other brain cancers.
Dr. Benedict Law, an associate professor of pharmacology in the Radiology Department at the Weill Cornell Medical College, is developing nanomaterials to more efficiently deliver oncology drugs to the brain, eliminate cancerous tumors and, ultimately, improve the survival rate of patients with DIPG tumors among other brain cancers.


A suite of Ginolis LFDA assembly solutions helps Abingdon Health to produce tens of millions of rapid tests per year. The combination of Abingdon Health’s long-established lateral flow manufacturing expertise and Ginolis LFDA technology enables the company to achieve the company’s mission: To improve life by making rapid results accessible to all.
Reproducible quality is vital for manufacturing accurate lateral flow tests. According to Abingdon Health, Ginolis certainly facilitates this to a large extent. The Ginolis LFDA system is flexible enough to work with the lateral flow test specifications of the company’s clients, enabling them to load multiple different tests onto the machines. This allows the company to offer flexible lateral flow manufacturing solutions in vastly increased volumes and at lower prices as part of their automated services.
Abingdon Health is a long-established lateral flow test manufacturer, who passionately pursues its mission to improve life by making rapid results accessible to all. With huge, 21st century technological advances in the speeds of online data transfer, modern society has become more and more expectant of immediate access to information.

“Ginolis LFDA technology alongside our long-established lateral flow manufacturing know-how is a great combination and enables us to work with many new customers on a whole array of different lateral flow tests,” says Oliver Gardner. “This ultimately helps us achieve our mission: To improve life by making rapid results accessible to all.”
Oliver Gardner, Chief Operating Officer
Abingdon Health

Heart attacks are one of the leading causes of death in the world. 3D Microfabrication can bring life science research a big step closer to the concept of regenerative medicine for the curing of diseases in this field.
Boston University scientists contributed to this goal with a microfluidic heart-on-a chip platform fabricated by Two-Photon Polymerization (2PP). This paves the way for fundamental studies of heart tissue and eventually enables the fabrication of tissue that can be implanted into the beating heart.
Boston University scientists contributed to this goal with a microfluidic heart-on-a chip platform fabricated by Two-Photon Polymerization (2PP). This paves the way for fundamental studies of heart tissue and eventually enables the fabrication of tissue that can be implanted into the beating heart.

Good Clean Love is advocating for the use of a 3D model of human tissue developed by MatTek as a more relevant alternative to testing women’s personal care products on animals.
In the US, personal lubricants are classified as medical devices by the Food and Drug Administration (FDA). As such, these intimate products are subject to regulatory requirements that include animal testing. But some argue that, beyond the ethical concerns, testing solely on animals is both outdated and detrimental to the health of female users because of the significant physiological differences.
As Strgar learned more about the limitations of animal testing, she got introduced to MatTek and their EpiVaginal™. A tissue model cultivated from human tissue that responds like in vivo human vaginal tissue and facilitates animal-free testing protocols that better mimic a woman’s anatomy.
Although available over the counter (OTC) in US drugstores, personal lubricants are classified as medical devices by the Food and Drug Administration (FDA). As such, these intimate products are subject to regulatory requirements that include animal-based skin sensitization toxicity testing, like in vivo rabbit vaginal irritation (RVI) and in vivo guinea pig maximization tests (Costin, 2020). But some argue that, beyond the ethical concerns, testing solely on animals is both outdated and detrimental to the health of female users because of the significant physiological differences.
“There are currently so many problems with women’s healthcare products. Partly, because they depend on archaic animal testing to approve new products. A lot of research shows that animal testing results do not provide an accurate reflection of safety.”
Wendy Strgar, CEO at Good Clean Love

The study of adult stem cells has increased exponentially because of recent technological innovations in the life sciences, especially within the field of bioprinting. The array of applications under consideration runs the gamut—from reversing baldness to managing diabetes to transplanting personalized bioprinted organoids.
With the latter, researchers are envisioning culturing a patient’s own adult stem cells to create viable organoids that could be transplanted into the patient with no need for the long-term immunosuppressant drugs they would have taken with a donated organ.
Ronawk enables large quantity production of adult stem cells thanks to its revolutionary T-blocks (tissue blocks). The technology includes biocompatible hydrogel scaffolds manufactured by CELLINK’s bioprinter LUMEN X + ™ where stem cells can grow.
The study of adult stem cells has increased exponentially because of recent technological innovations in the life sciences, especially within the field of bioprinting. The array of applications under consideration runs the gamut—from reversing baldness to managing diabetes to transplanting personalized bioprinted organoids.
With the latter, researchers are envisioning culturing a patient’s own adult stem cells to create viable organoids that could be transplanted into the patient with no need for the long-term immunosuppressant drugs they would have taken with a donated organ.
“The tissue block, or T-block, is a 3D scaffold that is expandable and modular to grow stem cells. The goal is to efficiently expand a patient’s own stem cells to engineer organoids or grafts that could be used in life-saving surgeries.”
A.J. Mellott, PhD
There is currently no way to repair damaged heart muscles from heart attacks, so many patients around the world wait on long heart transplant lists. Even for the lucky few who find a matching donor, there are more hurdles (i.e., organ viability, geographical distance, immune rejection). Therefore, heart disease remains the leading cause of death worldwide. According to the World Health Organization, 17.9 million lives are lost each year to cardiovascular diseases.
Dr. Carmine Gentile, leader of the Cardiovascular Regeneration Group at the University of Technology Sydney (UTS), announced in 2020 that his lab had “developed a technology that can 3D model and bioprint personalized hearts for transplantation, using the patient’s own stem cells so that there’s no risk of rejection.” Specifically, the technology identified the optimal conditions for cells to create blood vessels within bioprinted heart patches.
There is currently no way to repair damaged heart muscles from heart attacks, so many patients around the world wait on long heart transplant lists. Even for the lucky few who find a matching donor, there are more hurdles (i.e., organ viability, geographical distance, immune rejection). Therefore, heart disease remains the leading cause of death worldwide. According to the World Health Organization, 17.9 million lives are lost each year to cardiovascular diseases.
Dr. Carmine Gentile, leader of the Cardiovascular Regeneration Group at the University of Technology Sydney (UTS), announced in 2020 that his lab had “developed a technology that can 3D model and bioprint personalized hearts for transplantation, using the patient’s own stem cells so that there’s no risk of rejection.” Specifically, the technology identified the optimal conditions for cells to create blood vessels within bioprinted heart patches.
“We are using the patient’s own stem cells so that there’s no risk of rejection.”
Dr. Carmine Gentile

Karande and his team have developed a bioink made up of human endothelial cells, human pericyte cells and animal collagen. They used the CELLINK BIO X and Temperature Controlled Printhead to ensure that these constructs could be printed.
This combination of key elements enabled the cells to start communicating and more importantly begin forming a biologically relevant vascular structure within the span of a few weeks. Once blood vessels formed, nutrients and waste could be exchanged to keep the graft alive. The groups significant development highlights the vast potential of 3D bioprinting in precision medicine, where solutions can be tailored to specific situations.
Karande and his team have developed a bioink made up of human endothelial cells, human pericyte cells and animal collagen. They used the CELLINK BIO X and Temperature Controlled Printhead to ensure that these constructs could be printed.
This combination of key elements enabled the cells to start communicating and more importantly begin forming a biologically relevant vascular structure within the span of a few weeks. Once blood vessels formed, nutrients and waste could be exchanged to keep the graft alive. The groups significant development highlights the vast potential of 3D bioprinting in precision medicine, where solutions can be tailored to specific situations.
“…we could potentially even think of using this as a therapy for burn victims.”
Pankaj Karande
Associate Professor

An influential example is a study conducted by Dr. Nicola Crosetto’s lab at Karolinska Institutet, in which the I.DOT (Immediate Drop-on-demand Technology) liquid handler was used to automate and streamline a versatile technique (COVseq) for preparing multiplex DNA sequencing libraries from low-input samples with high accuracy, speed and significant reductions in liquid reagent volumes.
The study revealed that the COVseq technique can be easily applied to ongoing pandemic genomic surveillance and adapted to monitor other pathogens such as influenza viruses. In addition, an analysis of costs showed that the technique could be used to sequence thousands of samples per week at less than $10 per sample, including library preparation and sequencing costs.
An influential example is a study conducted by Dr. Nicola Crosetto’s lab at Karolinska Institutet, in which the I.DOT (Immediate Drop-on-demand Technology) liquid handler was used to automate and streamline a versatile technique (COVseq) for preparing multiplex DNA sequencing libraries from low-input samples with high accuracy, speed and significant reductions in liquid reagent volumes.
The study revealed that the COVseq technique can be easily applied to ongoing pandemic genomic surveillance and adapted to monitor other pathogens such as influenza viruses. In addition, an analysis of costs showed that the technique could be used to sequence thousands of samples per week at less than $10 per sample, including library preparation and sequencing costs.

“We were able to easily create new workflows for PCR and NGS applications and I highly recommend this system for all labs running high-throughput assays that require complex liquid dispensing schemes, as well as those aiming to lower assay costs by reducing reagent volumes.”
Nicola Crosetto, PhD
Monitoring the quality of water is of paramount importance for public health. SCIENION’s commitment to develop efficient, sensitive and robust multiplex tests for water quality analysis is reflected by the company’s involvement in various research projects such as microAQUA or “Rheines Wasser”.
In the EU-funded project microAQUA, twelve partners from eight countries collaborated to develop universal microarrays for the evaluation of fresh-water quality. MicroAQUA aimed to design and develop a microarray chip for the high-throughput detection of known and emerging pathogens (bacteria, viruses, protozoa and cyanobacteria) and to assess the water quality by monitoring the presence of select bioindicators such as diatoms.
The company developed the microarrays and provided the infrastructure and expertise for the production of the arrays and their analysis. This microarray was used for the analysis of the RNA extracted from environmental samples collected from fresh, brackish, marine and drinking water from different locations in six countries (Bulgaria, France, Germany, Ireland, Italy and Turkey).
In the EU-funded project microAQUA, twelve partners from eight countries collaborated to develop universal microarrays for the evaluation of fresh-water quality. MicroAQUA aimed to design and develop a microarray chip for the high-throughput detection of known and emerging pathogens (bacteria, viruses, protozoa and cyanobacteria) and to assess the water quality by monitoring the presence of select bioindicators such as diatoms.
The company developed the microarrays and provided the infrastructure and expertise for the production of the arrays and their analysis. This microarray was used for the analysis of the RNA extracted from environmental samples collected from fresh, brackish, marine and drinking water from different locations in six countries (Bulgaria, France, Germany, Ireland, Italy and Turkey).
“A key step on the preparation of the biochip is printing the microarray; here we can produce hundreds of small spots, less than a millimeter in diameter. The great advantage of this method is that we can get an answer almost immediately; in a day we can detect up to 150 different species in the water.”
Dr. Wilfried Weigel, VP of R&D at SCIENION
To address the need for personalized oncology drug testing, CEO Ishani Malhotra founded Carcinotech, the Edinburgh-based biotech company that is bioprinting patient-specific testing models with cells from biopsy samples. Find out how these ingenious researchers benefited from expert support from CELLINK, a BICO company.
Carcinotech produces 3D bioprinted tumor models using patient-derived cancer stem cells, primary cells and established cell lines. They developed their testing platform to enable rapid, ethical, sustainable and personalized drug testing. Because patients have different genetic makeups, a drug that works for one person might not work for others. Some chemotherapy drugs, temozolomide for example, will only work for 50% of patients. It is important to conduct individualized drug testing to ensure that the other 50% with a certain genetic mutation are identified. Then we can prescribe other drugs in the pipeline that will help them fight the cancer.
Why is Breast Cancer Awareness month important to you?
The annual breast cancer awareness campaign helps women and men (who also suffer from breast cancer) understand the importance of self-checking and scheduling lab tests to catch the disease early. Breast Cancer Awareness Month is also a chance to highlight the amazing progress researchers are making to develop more treatments options for breast cancer.
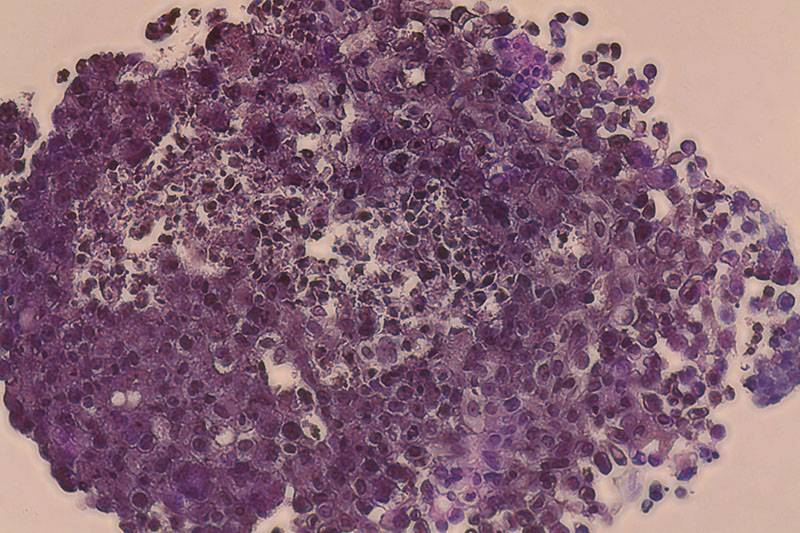
Breast cancer construct
”Carcinotech’s testing model makes it possible to screen thousands of potential cancer drug compounds in a high-throughput manner, with disease models that more accurately replicate human tissue and minimize the use of animals for preclinical testing.
Ishani Malhotra, CEO Carcinotech



